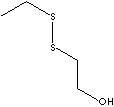
| ETHYL THIOETHANOL | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 110-77-0 |
|
| EINECS NO. | 203-802-0 | |
| FORMULA | C2H5SCH2CH2OH | |
| MOL WT. | 106.18 | |
| H.S. CODE | ||
|
TOXICITY |
||
| SYNONYMS | Ethyl 2-hydroxyethyl thioether; 2-Hydroxyethyl ethyl sulfide; | |
| 2-(Ethylthio)ethanol; beta-Ethylthioethanol; beta-Hydroxydiethyl sulfide; Ethyl beta-hydroxyethyl sulfide; Ethyl 2-hydroxyethyl sulfide; beta-ethylmerkaptoethanol; 2-(Ethylsulfanyl) Ethanol | ||
|
DERIVATION |
|
|
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | clear to pale yellow liquid | |
| MELTING POINT |
| |
| BOILING POINT | 180 - 185 C | |
| SPECIFIC GRAVITY |
1.015 - 1.025 | |
| SOLUBILITY IN WATER |
slightly soluble | |
| pH | ||
| VAPOR DENSITY | ||
| AUTOIGNITION |
| |
| NFPA RATINGS | Health: 2, Flammability: 1 Reactivity: 0 | |
| REFRACTIVE INDEX |
| |
| FLASH POINT |
77 C | |
| STABILITY | Stable under ordinary conditions. | |
|
GENERAL DESCRIPTION & APPLICATIONS |
||
|
Sulfate (also spelled sulphates in Euorpe) is any chemical compound related to sulfuric acid, formed by replacing one or both of the hydrogens with a metal or a radical. Sulfite is any salt of sulfurous acid, chemical species H2SO3, which is formed in aqueous solutions of sulfur dioxide. Sulfurous acid is a clear liquid with a strong sulfur aroma. Sulfide is a compound having one or more sulfur atoms in which the sulfur is connected directly to a carbon, metal, or other nonoxygen atom; for example, sodium sulfide, Na2S. Sulfur compounds are used in the synthesis of medicine and chemicals, manufacture of wood, pulp and paper. They are used in wine making, brewing, food preservation, metallurgy, engraving process, ore flotation, additive in making steel, bleaching, metal treatment and as an analytic reagent. Organosulfur compounds are widely used in refineries, steamcrackers, aromatic extraction and petrochemical manufacturing as they acts as hydrotreating catalysts, initial catalyst improvers, sulfur source and catalyst presulfiding. Sulfoxide (R2SO) is any of various organic sulfur compounds having the group -SO (sulfinyl group) whereas sulfone (RSOOR) with the group -SO2 (sulfonyl group). They are derived from oxidation of sulfides. They are widely used as solvent of both extraction and reaction as well as intermediates for the synthesis of textile chemicals and pharmaceuticals and agrochemicals. Dimethyl Sulfoxide is used as an effective extraction solvent and solvent improver for the separation of aromatic compounds (benzene, toluene and xylenes) from aliphatic hydrocarbons, and for fractionation of unsaturated components (olefins and alkynes) from saturated feedstock. It is used as a thermally stable medium for carrying out chemical reactions to make pharmaceuticals, agrochemicals (especially pyrethroid insecticides) paint and coating materials and biocides. Its pharmaceutical grade can be used as a as a local analgesic and anti-inflammatory agent. Sulfolane is used as a extraction as well as a reaction solvent. It is used to separate aromatic hydrocarbons (benzene, toluene and xylenes), It is used to separate n-propyl alcohol and sec-butyl alcohol. It is used to purify natural gas streams and for fractionation of fatty acids into saturated and unsaturated components. It is used as a reaction solvent for the preparation of aromatic sulfonic acids, pyridines, isocyanates and pharmaceuticals. It can also be involved in the halogen exchange process and polymerization process. It is used as a plasticizer and curing agent. Organosulfur compounds have diverse applications in the organic synthesis as organosulfur sources into target organic molecules in the manufacture of pharmaceuticals, adhesives, biocides, agrochemical products, lubricant and fuel additives for high pressure, surfactants, water treatment chemicals, dyes, flavors & fragrances, and photographic chemicals |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
clear to yellowish liquid | |
| CONTENT |
99.0% min | |
|
WATER |
0.5% max | |
|
SULFUR CONTENT |
29.0% min | |
| TRANSPORTATION | ||
| PACKING | 210kgs in drum | |
| HAZARD CLASS | ||
| UN NO. | ||
| OTHER INFORMATION | ||
| Hazard Symbols: XI, Risk Phrases: 36/38, Safety Phrases: 24/25 | ||